Mechanobiology
Development of methods to study mechanical aspects of cells and tissues
Machine learning to classify cells on the basis of their mechanical properties
Doctoral researcher: Ophélie Thomas Chemin
Supervisor: Etienne Dague, Childérick Severac
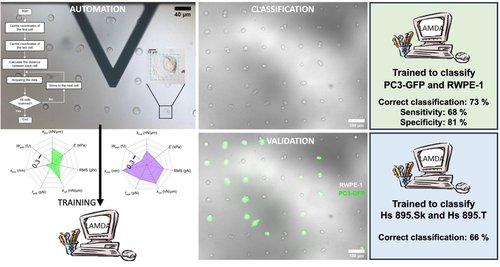
We trained a machine learning algorithm, based on fuzzy logic, called LAMDA,18−20 to recognize two cell types. By posing the problem of classification, bio-AFM can be used as a real-world technique for diagnostic purposes, for example. Indeed, a diagnosis starts with a population of unknown cells, and it is then necessary to find among this population the cells exhibiting a pathological signature on the basis of their biomechanical features. In this context, the statistical comparison is not valid because even if the two conditions are statistically different, it is often impossible, on the basis of a single measurement or a series of measurements, to attribute a cell to one or the other condition. This is where our work differs from most studies involving the measurement of mechanobiological properties by AFM. The overall concordant classification rate between the algorithm and the fluorescent validation was 73%, among which 68% of cancerous cells and 81% of normal epithelial cells were correctly identified.
Publications:
Thomas - - Chemin O., Severac C., Moumen A., Martinez-Rivas A., Vieu C., Le Lann M-V, Trévisiol E., Dague E. 2024. Automated bio-AFM generation of large mechanome data set and their analysis by machine learning to classify prostatic cell lines, ACS applied materials and Interfaces 16, 34, 44504–44517 https://doi.org/10.1021/acsami.4c09218
Thomas- -Chemin O., Janel S., Boumehdi Z., Séverac C., Trévisiol E., Dague E.*, Duprés V.* 2024. Advancing high-throughput cellular AFM with automation & artificial intelligence, ACS Nano in Press
Collaborations: RESTORE (Childérick Severac), LAAS (DISCO : Marie-Véronique Lelann), CIC IPN Mexico (Adrian Martinez-Rivas)
Fundings : Agence Nationale de la Recherche (ANR) AutoBioTip - ANR-20- CE42-0017, ECOS Nord ECOS M15P02 Campus France
Bio-AFM robotisation based on automation and image recognition
Research Engineer : Zeyd Boumehdi / Doctoral researcher: Ophélie Thomas Chemin
Supervisor: Etienne Dague, Emmanuelle Trévisiol (LAAS CNRS - TBI)

Nanomechanical properties of cells could be considered as cellular biomarkers. The main method used to access the mechanical properties is based on nanoindentations measurements, performed with an operator manipulated Atomic Force Microscope (AFM) which is time-consuming, and expensive. This is one of the reasons preventing the transfer of AFM technology into clinical laboratories. In this work we develop methodologies which includes an algorithm able to automatically move the tip onto a single cell and through several cells to record force curves combined with a smart strategy of cell immobilization.
Publications:
Severac C., Proa-Coronado S., Formosa-Dague C., Martinez-Rivas A., and Dague E. 2021. Automation of bio-atomic force microscope measurements on hundreds of C. albicans cells, Journal of Visualized Experiments 170, e61315, doi:10.3791/61315
Proa-Coronado S., Severac C., Martinez-Rivas A., and Dague E. 2020. Beyond the paradigm of nanomechanical measurements on cells using AFM: an automated methodology to rapidly analyze thousands of cells. Nanoscale Horizons, 5, 131-138, https://pubs.rsc.org/en/content/articlelanding/2020
Collaborations : RESTORE (Childérick Severac) , CIC IPN (Mexico Adrian Martinez-Rivas)
Study of antibacterial action mechanisms of silver nanoparticles by in situ microscopy - BioAFM
Doctoral researcher: Maria Clara Müller de Andrade
Supervisor: Etienne Dague, André Galembeck
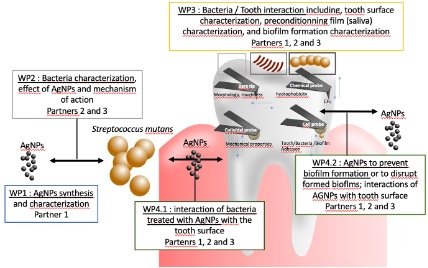
This project studies the action of silver nanoparticles in inhibiting biofilm formation on inorganic surfaces during the caries-originating process, and on surface colonization by antibiotic-resistant bacteria that cause hospital-acquired infections. In situ techniques based on atomic force microscopy will be used, and methodologies will be developed for determining quantitative parameters of bacterial/substrate and nanoparticle/bacterial adhesion.
Publications:
Müller de Andrade M.C., Rosenblatt A., Galembeck A. 2022. Silver nanoparticles penetration in dentin: Implications for long-term caries arrestment, Materialia Volume 24, August 2022, 101489 https://doi.org/10.1016/j.mtla.2022.101489.
Collaborations : André Galembeck: Université fédérale de Pernambouck Recife, Brasil
Fundings: COFECUB / Campus France : Me 1019/24 / 50567WB
Mechanics in cell-cell interactions
Doctoral researcher: Easter Ndlovu
Supervisors: Etienne Dague, Tanya Dahms
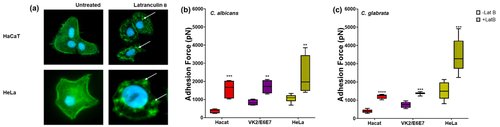
The mechanical properties of malignant cells, such as adhesiveness and viscoelasticity, which contribute to cellular invasion and migration are different from those of noncancerous cells. To understand host invasion and its relationship with host cell health, we probed the interaction of Candida spp. with cancerous and noncancerous human cell lines using atomic force microscopy in the single-cell force spectroscopy mode. There was significant adhesion between Candida and human cells, with more adhesion to cancerous versus noncancerous cell lines. This increase in adhesion is related to the mechanobiological properties of cancer cells, which have a disorganized cytoskeleton and lower rigidity.
Collaboration: University of Regina Canada (Tanya Dhams)
Publication:
Ndlovu E., Malpartida L., Sultana T., Dahms T. E. S., Dague E. 2024. Host cell geometry and cytoskeletam organization governs Candida-host cell interaction at the nanoscale range; ACS applied materials and Interfaces 15, 44, 50789–5079, https://pubs.acs.org/doi/10.1021/acsami.3c09870
AFM for single cell transcriptome analysis
Doctoral researcher: Koutayba Saada
Supervisor: Etienne Dague, Laurent Malaquin
Description of the project
Impact of mechanics on Microphage phagocytisis
Post-Doctoral researcher: Delphine Bretonnière
Associated researchers: Laurent Malaquin, Christophe Thibault, Renaud Poincloud (IPBS)
Macrophages, key cells in our innate immune system, ensure immune surveillance and homeostasis by ingesting bacteria or apoptotic cells. This process, known as phagocytosis, involves dynamic reorganization of the actin cytoskeleton and the application of mechanical forces to the particles to be ingested. Despite the importance of this function, the molecular mechanisms involved in force generation during phagocytosis are extremely poorly documented. This is mainly due to the lack of a simple approach for assessing these forces.
We therefore propose to develop an innovative method called Compression Force Microscopy to measure with high precision the amplitude, direction and dynamics of the compressive and tensile forces generated on the micropillars during phagocytosis. This interdisciplinary project, combining cell biology, microfabrication and cutting-edge imaging, will provide a better understanding of this key, yet little-known, mechanism of our immune system, and help identify potential new targets for modulating it in pathological situations. We aim to extend this method to the mechanical study of epithelial monolayers and study forces at cell junctions.
Collaboration: IPBS (Renaud Poincloud, Javier Rey-Barroso), Claire Bigot
Fundings: ANR NanoTopoAdhesion, ITMO Cancer PCSI 2022 NanoTopoTAMs, CNRS Prime80
Cellular detection of nano-topographies: Implication for the infiltration of tumor-associated
Doctoral researcher: Océane Dewingle
Supervisors: Christophe Thibault, Renaud Poincloud (IPBS)
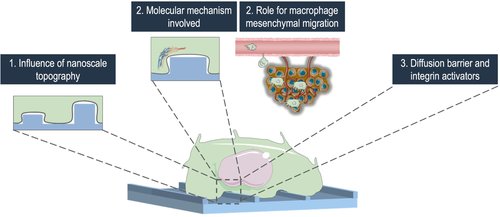
Macrophages are essential innate immune cells present in all body tissues. While they have an important role in organism defense, their massive infiltration into diseased tissues can favor the progression of the pathology such as inflammation and cancer. Understanding how macrophages navigate through their environment, particularly how they recognize and respond to environment various shapes and topographical patterns, remains unclear. This study focuses on podosomes, adhesion structures in macrophages essential for their migration, and their dynamics in response to topographies.
We developed a nano-fabrication strategy to create topographies of controlled dimensions on glass substrates, ranging from a few nanometers to hundreds of nanometers in height with various shapes and widths. Preliminary results show that macrophages and their podosomes are able to response to topographies at a scale as small as 20 nanometers of height.
This highlights the significance of considering nanoscale environmental patterns in understanding cell behavior and now allow us to investigate the mechanisms involved in this recognition. They also pave the way for the development and the use of new and more complex forms of topography.
Publications:
- M. Portes et al. Nanoscale architecture and coordination of actin cores within the sealing zone of human osteoclasts, eLife 11:e75610 (2022) https://doi.org/10.7554/eLife.75610
- E. Desvignes et al. Nanoscale Forces during Confined Cell Migration, Nano Letters 2018 18 (10), 6326-6333, https://doi.org/10.1021/acs.nanolett.8b02611
Collaborations : IPBS (Renaud Poincloux), Institut Curie (Patricia Bassereau), IINS (Olivier Rossier)
Fundings: ANR NanoTopoAdhesion, ITMO Cancer PCSI 2022 NanoTopoTAMs