Polymeric free-standing architectures for cell colonization
The realization of 3D architectures for the study of cell growth, proliferation, and differentiation is a task of fundamental importance for both technological and biological communities involved in the development of biomimetic cell culture environments. Here we report the fabrication of 3D freestanding scaffolds, realized by multiphoton direct laser writing and seeded with neuroblastoma cells, and their multitechnique characterization using advanced 3D fluorescence imaging approaches. The high accuracy of the fabrication process (≈200 nm) allows a much ner control of the micro- and nanoscale features compared to other 3D printing technologies based on fused deposition modeling, inkjet printing, selective laser sintering, or polyjet technology. Scanning electron microscopy (SEM) provides detailed insights about the morphology of both cells and cellular interconnections around the 3D architecture. On the other hand, the nature of the seeding in the inner core of the 3D scaffold, inaccessible by conventional SEM imaging, is unveiled by light sheet uorescence microscopy and multiphoton confocal imaging highlighting an optimal cell colonization both around and within the 3D scaffold as well as the formation of long neuritific extensions. The results open appealing scenarios for the use of the developed 3D fabrication/3D imaging protocols in several neuroscientific contexts.
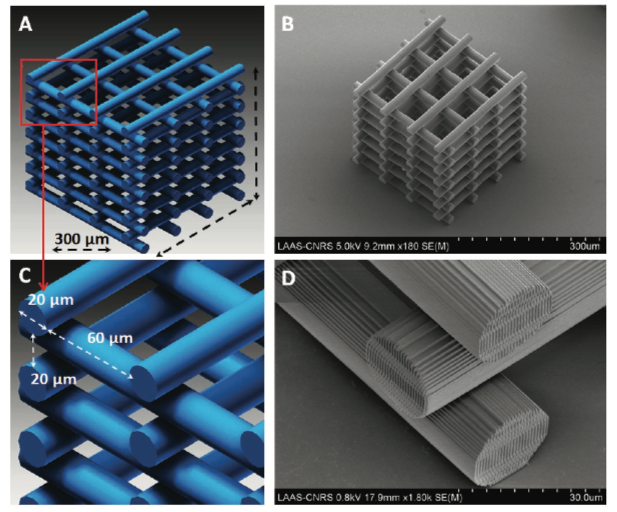
A) CAD design of the 3D scaffold; B) SEM micrograph of the 3D scaffold; C) close-up on the micrometer-scaled cylindrical subunits of the CAD design; D) close-up on the micro- and nanoscale features of the 3D scaffold.
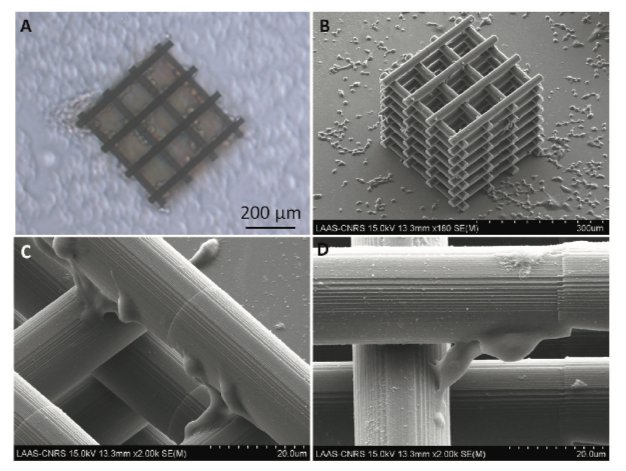
Optical A) and SEM B) micrographs of the 3D scaffold seeded with N2A cells; C) close-up on the cellular morphology and adhesion around the 3D scaffold cylindrical subunits; D) N2A cells typical interconnection along the z-axis linking cylindrical subunits at different z-heights.

